New drugs on the block
New drugs on the block
the past few years have seen an alarming increase in the number of malaria cases. The World Health Organisation ( who ) puts the annual number of cases between 300-500 million, and deaths between 1.5-2.7 million. Controlling the disease has become difficult due to insecticide resistance in the mosquito and difficulties in implementation of vector control programmes, among other reasons. In fact, there are fewer tools to control malaria now than there were 20 years ago.
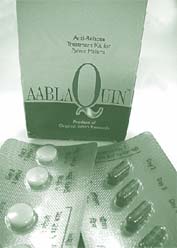
As the struggle to control the disease continues, many research institutes have come up with new drugs. One of them is the Central Drug Research Institute ( cdri ), Lucknow, which has come out with a drug called cdri compound 80/53, also known as Aablaquin. The drug is less toxic than Primaquine, which is used widely to cure relapsing malaria caused by Plasmodium vivax . “The parasite is present in both the blood and tissue such as liver,” says O P Asthana of cdri . Chloroquine, another commonly-used drug kills the parasite present in the blood, but fails to affect the pathogen in the tissues which can then reproduce and cause the relapse. Primaquine, though effective, has been found to be toxic. This toxicity can lead to side-effects such as gastrointestinal disorders and breakdown of red blood cells leading to acute anaemia. Moreover, the drug cannot be given to small children and pregnant women. Trials conducted on nearly 700 patients by the New Delhi-based Malaria Research Centre found that the relapse rate for Chloroquine was the highest (41 per cent) followed by Aablaquin (30 per cent) and Primaquine (28 per cent).
“Though the relapse rate of the new drug is slightly higher than that of Primaquine, the lower toxicity is significant,” says Asthana. “The toxicity of the drug is lower as it has been purified and the toxic components have been removed,” says Ram Pratap, a scientist with cdri . The new drug has been launched by Nicholas Piramal India Limited, Mumbai, and called Aablaquin. Sanjay Chitkara of Nicholas Piramal says that since the product was launched after the malaria season, they do not have actual sales figures but most physicians feel that the product is very good.
However, experts have a word of caution. Says V P Sharma, former director of Malaria Research Centre and now with the who ’s Roll Back Malaria Programme: “More clinical trials must be carried out to assess the risks of the drug before it is given indiscriminately to patients.” Giving an example, he says: “Trials of this drug have not been conducted on people deficient in the enzyme g6pd , lack of which can cause the breakdown of the red blood cells when given the drug.”
Another drug called a , b -Arteether, earlier launched by cdri, is being used to cure patients suffering from Plasmodium falciparum, a different strain of pathogen. The drug is prepared from artimisinin, a chemical derived from a Chinese plant called Artimisia annua . Scientists at the Central Institute of Medicinal and Aromatic Plants ( cimap ), Lucknow, have helped cdri scientists by successfully developing a variety of the plant. “Work is being carried out to develop plants which have high artimisinin content,” says A K Singh, a scientist with cimap .
The product is available in the form of an injection and is marketed by Themis Chemicals, a Mumbai-based pharma







